We at LM-Dental™ are dedicated to meet the high quality standards for developing and manufacturing first class products which make the dental care safe efficient and pleasant. We are proud to inform that LM-Dental™ has been granted a new EU-certificate and all our periodontal hand instruments now conform to the MDR class Ir standards. You will notice that the CE-mark on the product, product packages, and on any related marketing materials for the periodontal instruments now includes an identification number for the notified body. Please check our current certificates and declarations here.
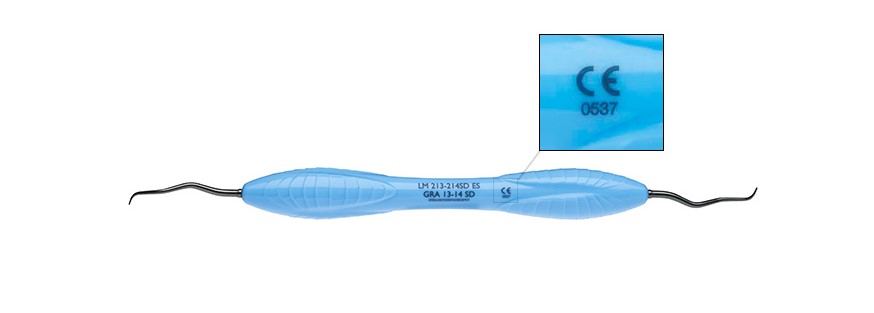
Above an example of the new CE-mark for the products under EU MDR Ir class.
A notified body is an organisation designated by an EU country to assess the conformity of certain products before being placed on the market. The European Commission publishes a list of such notified bodies.
The periodontal hand instruments are our first product group moving to the EU MDR Class Ir and we will continue the transition project with next-in-line products groups.
We at LM-Dental™ are really proud to develop, manufacture and market top quality products that meet the highest standards! We hope that you feel the same about LM-Dental™ products!






